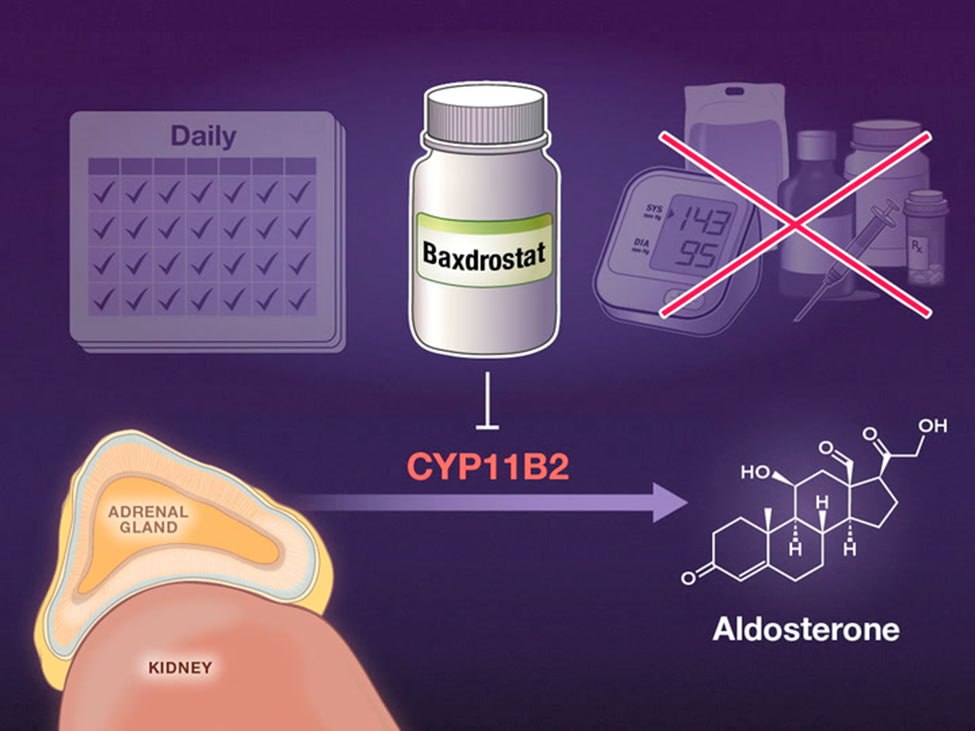
AstraZeneca said on Tuesday its experimental drug baxdrostat significantly lowered blood pressure during a 24-hour window of monitoring in a late-stage trial of patients with treatment-resistant hypertension.
The company said the drug met the main goal of the study when compared to placebo at the end of 12 weeks. Patients in the trial had received 2 milligrams of baxdrostat or placebo on top of standard care.
AstraZeneca has previously said that it expects to file for regulatory approval of the drug before the end of the year.
Baxdrostat is currently being investigated for four indications, including chronic kidney disease and prevention of heart failure.