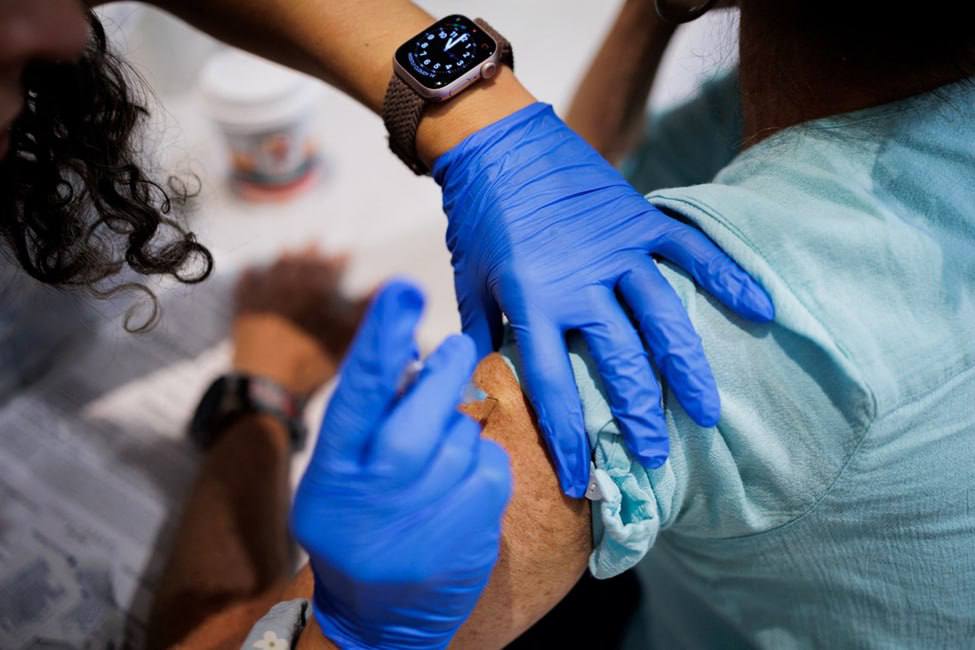
The American Academy of Family Physicians said on Monday that it recommends all adults over the age of 18, children and pregnant women to receive COVID-19 vaccines, a contrast to the U.S. government’s narrowed approvals for the shots.
Its suggested immunizations for COVID are in line with the pediatric physician’s group, The American Academy of Pediatrics’ recommendations from last month.
They contrast with the U.S. Food and Drug Administration which two weeks ago approved the updated vaccines for people with health conditions and all people aged 65 and older.
Health Secretary Robert F. Kennedy said in May the U.S. no longer recommends routine COVID-19 shots for healthy kids and pregnant women, prompting medical organizations and several states to formulate their own vaccine recommendations.
Previously, all Americans had access to the shots.
The AAFP recommends all children aged six to 23 months be vaccinated against COVID-19 and employs a risk-based single dose approach for children and teens between two and 18 years old.
It also said vaccinations are especially important for people aged 65 or older, those at risk for severe COVID-19 infection, and those who have never received a shot.
“History shows us that vaccines have eradicated diseases that were disabling and deadly in the past, and we can keep it that way, if we continue to vaccinate,” said Margot Savoy, chief medical officer of the AAFP, adding the group “stands with our members and public health partners to promote vaccine confidence and uptake.”
The AAFP also gave recommendations for vaccinations against respiratory syncytial virus and influenza, which were in line with the U.S. Centers for Disease Control and Prevention.
(This story has been corrected to show that the age range for young children to be vaccinated is six months to 23 months, not six months to 18 months, after the physicians group corrected the statement, in paragraph 6)